
Given the equilibrium: HCN(aq) + H2O(l) ←→ H3O+(aq) + CN-(aq) ΔH >0 Ka = 4.0 x10-10 What happens to the concentration of hydrogen cyanide when the following stresses are placed on the system at equilibrium? NaCl is added -> Stays the Same but HCN (aq) + KOH (aq)-> H2O (l) + KCN (aq) write the net ionic equation, including the phasesĬonvert the following into a balanced equation: Liquid disilicon hexachloride reacts with water to form solid silicon dioxide, hydrogen chloride gas, and hydrogen gas. carbon, hydrogen, and oxygen.*** Nucleic acids are the building blocks of proteins.įor the following chemical reaction. Here is the equation: KCN(aq) + HCl(aq) -> KCl(aq)+ HCN(g) If a sample of 0.140 g of KCN is treated with an excessĪll biomolecules are made up of the elements carbon, hydrogen, nitrogen, oxygen, and sulfur. 502 O E.When potassium cyanide (KCN) reacts with acids, a deadly poisonous gas, hydrogen cyaninde (HCN) is given off. Click here👆to get an answer to your question ️ Among the following the total number of polar molecules are.

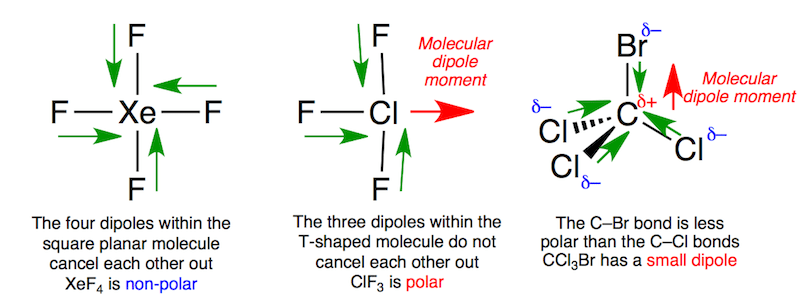
For diatomic molecules, there is only one bond, so its bond dipole moment determines the molecular polarity. Tetrahedral, AX4 net The dipole moment (μ) is the calculation of the net molecular polarity at either end of the molecular dipole, which is the magnitude of the charge Q times the distance r between the charges. It was the first discovered binary compound of a noble gas. Calculate the percentage ionic character. 2, according to the chemical equation: Xe + 2 F. This molecule does not have a permanent dipole moment (i. Of sulfur's total of six valence electrons, two form a lone pair. XeF4 is a nonpolar molecule so that its dipole moment is 0D (Zero). Is XeF4 dipole-dipole? XeF4 is non polar.


 0 kommentar(er)
0 kommentar(er)
